Spontaneous Abnormal Involuntary Movements in First-Episode Schizophrenia and Schizophreniform Disorder: Baseline Rate in a Group of Patients From an Irish Catchment Area
Abstract
Objective: This study investigated the rate of spontaneous abnormal involuntary movements in a group of patients presenting with a first episode of schizophrenia or schizophreniform psychosis. Method: Seventy-nine patients with a first episode of schizophrenia or schizophreniform psychosis who presented to a catchment area psychiatric service over a 3-year period, and who were neuroleptic-naive or had been medicated for less than 1 month, were examined for the presence of involuntary movements with use of the Abnormal Involuntary Movement Scale.Results: Six patients (7.6%) had spontaneous dyskinesia as defined by the criteria of Schooler and Kane, and nine other patients had mild orofacial involuntary movements. The patients with spontaneous dyskinesia had completed significantly fewer years of education than the patients without dyskinesia. Spontaneous involuntary movements were unrelated to age at presentation for treatment. Conclusions: Spontaneous abnormal involuntary movements were evident among a proportion of patients with first-episode schizophrenia or schizophreniform psychosis at baseline presentation and were associated with reduced educational attainment. This finding supports previous suggestions that abnormal involuntary movements in schizophrenia may be related to the pathophysiology of the illness and therefore cannot be attributed entirely to the adverse effects of neuroleptic medication. Am J Psychiatry 1998; 155: 1202-1206
Tardive dyskinesia has long been considered to be a side effect of neuroleptic medications (1, 2). The fact that it is both common and potentially irreversible (3, 4) makes it perhaps the most serious long-term side effect of these drugs, which are one of the mainstays of treatment of schizophrenia and other psychotic disorders.
An alternative perspective is that abnormal involuntary movements are not simply a side effect of treatment but may be, at least partially, an inherent part of some psychotic illnesses (5, 7). Reports of abnormal involuntary movements in schizophrenia date almost from the first description of the disorder itself. The abnormal involuntary movements in dementia praecox described by Kraepelin (8) are indistinguishable from the movements we now term tardive dyskinesia. Retrospective examination of case records from the preneuroleptic era (9, 10) suggests a movement disorder rate of 15%–28%, which is similar to the reported rate of 15%–20% for tardive dyskinesia in the postneuroleptic era (3, 11, 12).
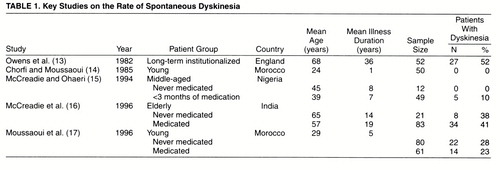
Studies on the frequency of spontaneous dyskinesia (13-17) are summarized in table 1. These studies, with the exception of the one by Moussaoui and colleagues (17), tend to show an increasing rate of spontaneous dyskinesia with increasing age and chronicity of illness. The highest rate was reported by Owens et al. (13) in a sample of long-term institutionalized patients, which may have a selection bias for higher rates of movement disorders (18). Studies of groups of noninstitutionalized patients report somewhat lower but widely varying rates that are not fully explained by difference in age (14-17).
A fundamental issue is whether such movements are present at the time of first presentation of the illness or develop over time, either in relation to or independent of exposure to neuroleptic medication. In a study of first-episode schizophrenia/schizoaffective disorder, Chatterjee and colleagues (19) found that only one of 89 patients evidenced abnormal involuntary movements. We undertook a study to ascertain the rate of spontaneous abnormal involuntary movements at baseline presentation among Irish patients with first-episode schizophrenia/schizophreniform psychosis from a defined urban catchment area population.
METHOD
The subjects were drawn from an ongoing prospective study of first-episode psychosis being carried out in Cluain Mhuire, a south Dublin catchment area that provides community-based mental health care for a geographically defined region with a population of 165,000 persons. Patients who came to the psychiatric service and met the DSM-III-R criteria for schizophrenia or schizophreniform psychosis were assessed at the time of presentation. “First episode” was defined as the first presentation of a patient with acute psychotic symptoms to a psychiatric service. The purpose and nature of the study were explained to the patients, and informed consent was obtained.
Patients were examined for the presence and severity of involuntary movements by one of the investigators (M.G.) using the Abnormal Involuntary Movement Scale (AIMS), which assesses involuntary movements in seven body areas and has been described in detail elsewhere (20). The presence of spontaneous dyskinesia was determined with use of the criteria of Schooler and Kane (21), which require that a patient have mild involuntary movements in at least two body areas or moderate involuntary movements in one body area to be classified as a “case.” After the AIMS examination, demographic and clinical data were compiled by interview and review of clinical case notes.
Statistical analyses were performed with the Statistical Package for the Social Sciences. Between-group differences were examined with the use of two-tailed independent t tests and Fisher’s exact test. The relationship of sociodemographic and clinical variables to dichotomous and linear AIMS measures was examined by means of multiple logistic and linear regression, respectively.
RESULTS
Seventy-nine patients with a first episode of psychosis who met the DSM-III-R criteria for schizophrenia or schizophreniform psychosis were assessed over the period of the study. Of these, 50 (63%) were male and 29 were female. Their mean age was 27.7 years (SD=9.7). Forty-nine of these patients were neuroleptic-naive at the time of assessment; the remaining 30 had been medicated for less than 1 month, either having been started on medication before referral by their primary health care physicians or being too ill to cooperate with assessment before treatment began. Seventeen patients (22%) also met the DSM-III-R criteria for drug or alcohol abuse or dependence in the past month. Four patients had comorbid physical illness: one had partial deafness, another had a congenital hand deformity, the third had a history of head injury, and the fourth was receiving interferon treatment for nonmetastatic malignant melanoma. Seventy-seven patients were Caucasian, and two patients were of mixed racial origin.
The mean total AIMS score for the group was 1.6 (SD=2.2, range=0–12). When the criteria of Schooler and Kane were applied, six patients were found to have spontaneous dyskinesia. Five of these were neuroleptic-naive, and one had been treated with neuroleptics for 1 week; all were of Irish origin and free of comorbid physical illness. Two of these patients with spontaneous dyskinesia had comorbid cannabis and alcohol dependence. The topography of their involuntary movements was primarily orofacial; three patients had mild orofacial movements of the tongue, jaw, and lips, and two patients evidenced a mixed topography with jaw and tongue movements (one having moderate severity) in conjunction with mild upper limb movements. One patient had mild upper and lower limb movements only. A further nine patients had mild involuntary movements of one orofacial region but did not meet the Schooler and Kane criteria for spontaneous dyskinesia. The four patients with comorbid physical illness did not have mild involuntary movements in any body area. The rate of spontaneous schizophrenia/schizophreniform psychosis was 7.6% (N=6 of 79; 95% confidence interval=2.8%–15.8%). The rate in the neuroleptic-naive group was 10.2% (N=5 of 49; 95% confidence interval=3.4%–22.2%). Exclusion of patients with current alcohol or drug abuse or dependence had little influence on either rate: 6.5% (N=4 of 62) in the total group and 9.8% (N=4 of 41) in the neuroleptic-naive group.
The mean age at presentation did not differ between the patients with spontaneous dyskinesia and those without (mean=25.3 years, SD=11.6, and mean=27.9 years, SD=9.6, respectively; t=0.63, df=77, p=0.53). There was no significant difference in gender distribution between the group of patients with spontaneous dyskinesia (three male and three female) and the group without (47 male and 26 female) (p=0.66, Fisher’s exact test).
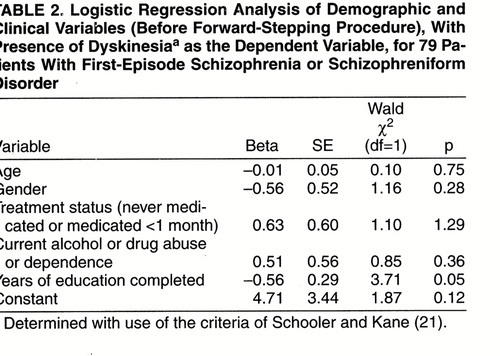
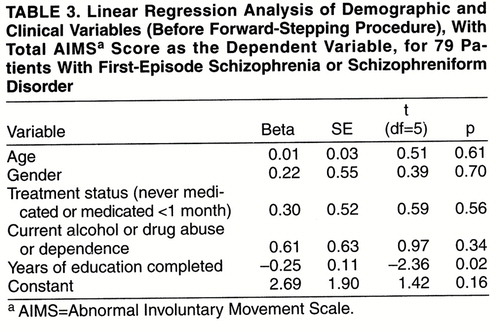
Patients with spontaneous dyskinesia had completed significantly fewer years of education (mean=11.3 years, SD=2.3) than patients without spontaneous dyskinesia (mean=13.3 years, SD=2.4) (t=2.15, df=77, p=0.03). However, in a logistic regression (table 2) in which the presence of spontaneous dyskinesia was the dependent variable and age, gender, years of education, treatment status, and current drug or alcohol abuse or dependence were the independent variables, the overall regression model was not significant (-2 log likelihood χ2=8.21, df=5, p=0.15). When we went on to do a stepwise logistic regression using the same variables, years of education discriminated between the groups (Wald χ2=4.26, df=1, p=0.04, after the forward-stepping procedure). However, when we used a Bonferroni correction for the five variables considered in the regression, this failed to reach the required level of significance (corrected p=0.20). In a multiple linear regression (table 3) with total AIMS score as the dependent variable and the same independent variables as above, the overall regression model was not significant (F=1.41, df=5, 73, p=0.23, R2=2.6%). After forward stepping, years of education was the only variable related to the total AIMS score (F=4.93, df=1, 77, p=0.03, R2=4.8%). However, again this did not meet the required level of significance after Bonferroni correction (corrected p=0.15).
DISCUSSION
The principal finding of this study was a baseline rate of 7.6% for spontaneous dyskinesia in a group of patients with first-episode schizophrenia or schizophreniform disorder presenting to a defined catchment area psychiatric service over a 3-year period, which is higher than the rate (1.1%) reported by Chatterjee and colleagues (19) in their first-episode group. As pointed out by these investigators (22), an important methodological issue in such studies is sampling technique, since previous studies in the United States found lower rates of tardive dyskinesia among patients in voluntary hospitals (13.3%) than among patients in state hospitals (36.1%) (23). In contrast, the organization of psychiatric services in Ireland is such that patients are treated in centers that serve defined geographical regions, and these patients would therefore be more representative. The lower rate of spontaneous involuntary movements in our first-episode study group than the 15%–28% found by Fenton and colleagues (9), in a retrospective study of patients from the preneuroleptic era, may be explained by differences in the severity criteria used to define “caseness” or by the fact that movements were assessed a mean of 4.5 years after first admission in their study.
Our finding that the patients with spontaneous dyskinesia were mainly from the never-medicated group (10.2%, N=5 of 49), rather than from the group who were minimally medicated before assessment (3.3%, N=1 of 30), might suggest a possible underestimate of the rate of spontaneous dyskinesia due to the phenomenon of masking caused by the presence of antipsychotic-induced parkinsonism; this possibility was not formally assessed and therefore could not be controlled for in the present study. Thus, the higher rate of 10.2% found in the 49 neuroleptic-naive patients may be a more accurate estimate.
This finding seems at odds with the findings of McCreadie and Ohaeri (15) in their Nigerian study, where the rate was 0% for spontaneous dyskinesia in 12 never-medicated patients and 10% in 49 patients medicated for less than 3 months. However, given the modest size of their never-medicated group and an average rate of 5% for spontaneous dyskinesia (11), this may well have been a chance finding. Since Schooler and Kane (21) define dyskinesia as spontaneous if the cumulative drug exposure is less than 3 months, combining the two groups in the McCreadie and Ohaeri study gives a rate of 8.2% in 61 patients, which is similar to our finding and may be a closer reflection of the “true” rate of spontaneous dyskinesia.
The mean total AIMS score for our six patients with spontaneous dyskinesia was 7.3, indicating moderate dyskinesia according to the criteria of Gardos and Casey (24). This is similar to the mean total AIMS score of 7.5 previously found for 15 patients with tardive dyskinesia in a group of 60 day patients from the same catchment area who had received long-term treatment with neuroleptics (25). Thus, the severity of spontaneous involuntary movements in first-episode patients is similar to that in chronically ill, medicated patients with tardive dyskinesia.
Although our finding that patients with spontaneous dyskinesia had completed fewer years of education was not significant in the regression models, Fenton et al. (9) previously reported an association between spontaneous dyskinesia and both a more malignant course and lower IQ among patients with schizophrenia. There is an extensive literature regarding the association between tardive dyskinesia and cognitive function. Most authors (13, 26-30) but not all (31, 32) report that patients with tardive dyskinesia show poorer performance on neuropsychological testing when compared with patients without such movements. An important question is whether tardive dyskinesia goes hand-in-hand with cognitive dysfunction or cognitive dysfunction antedates tardive dyskinesia. While the literature is contradictory in this regard (33, 34), if one considers a lower number of years of education to be indicative of poorer cognitive function (35), our data, albeit modest due to the study group size, indicate that patients who are neuroleptic-naive and who have lower educational attainment are more likely to demonstrate abnormal involuntary movements at presentation for treatment. This would suggest that factors relating to the origins of schizophrenia itself may be intimately related to spontaneous dyskinesia. Whether patients with lower educational attainment are at increased risk over time of developing tardive dyskinesia can only be answered by prospective follow-up studies.
Our finding of no relation between the presence of spontaneous dyskinesia and age in our subjects is not surprising in a group of relatively young first-episode patients that is homogeneous in age. The association between increasing age and involuntary movements is perhaps the most robust finding across studies in samples with different ages (11, 19, 36, 37) and merits consideration as a potential confounding factor when one is comparing rates among study groups or examining clinical correlates. Similarly, the lack of a relationship between spontaneous dyskinesia and gender is not surprising in this first-episode group. In previous studies on tardive dyskinesia, some investigators have detected such a difference (38, 39), while others have not (40). It has been suggested that studies finding a higher rate of involuntary movements in female subjects generally involve older populations; thus, the gender effect may itself be age dependent (11, 41). However, the modest number of patients with spontaneous dyskinesia in this study may have limited the detection of any gender difference.
Spontaneous dyskinesia was more common at baseline presentation among patients with first-episode schizophrenia from a defined catchment area population than was found previously in the first-episode group of Chatterjee and colleagues (19). This adds weight to the argument that involuntary movements in schizophrenia may be at least in part intrinsic to the pathophysiology of the illness rather than a side effect of its treatment. The lower number of years of education completed by patients with spontaneous involuntary movements, if considered indicative of poorer cognitive function, suggests that associations between involuntary movements and poorer cognitive function may antedate the onset of the illness and may also be independent of treatment with neuroleptic drugs. This emphasizes the clinical and medicolegal importance of monitoring patients for the presence of spontaneous involuntary movements at the time of first presentation to a psychiatric service.
Received April 23, 1997; revisions received Sept. 24, 1997, and Feb. 3, 1998; accepted March 24, 1998. From the Theodore and Vada Stanley Research Unit, Cluain Mhuire Services, Hospitaller Order of St. John of God; and the Royal College of Surgeons in Ireland, Dublin.. Address reprint requests to Dr. O’Callaghan, Cluain Mhuire Family Centre, Newtownpark Avenue, Blackrock, County Dublin, Ireland. Supported by the Theodore and Vada Stanley Foundation.The authors thank Dr. Pak Sham for his comments.
 |
 |
 |
1. Granacher RP Jr: Differential diagnosis of tardive dyskinesia: an overview. Am J Psychiatry 1981; 138:1288–1297Link, Google Scholar
2. Fann WE: Tardive dyskinesia and other drug induced movement disorders, in Tardive Dyskinesia: Research and Treatment. Edited by Fann WE, Smith RC, Davis JM, Domino EF. Jamaica, NY, Spectrum Publications, 1980, pp 216–232Google Scholar
3. Gerlach J, Casey DE: Tardive dyskinesia. Acta Psychiatr Scand 1988; 77: 369–378Google Scholar
4. Uhrbrand L, Faurbye A: Reversible and irreversible dyskinesia after treatment with perphenazine, chlorpromazine, reserpine and electroconvulsive therapy. Psychopharmacologica 1960; 1:408–418Crossref, Google Scholar
5. Crow TJ, Cross AJ, Johnston EC, Owen F, Owens DGC, Waddington JL: Abnormal involuntary movements in schizophrenia: are they related to the disease process or its treatment? are they associated with changes in dopamine receptors? J Clin Psychopharmacol 1982; 2:336–340Google Scholar
6. Waddington JL: Schizophrenia, affective psychoses, and other disorders treated with neuroleptic drugs: the enigma of tardive dyskinesia, its neurobiological determinants, and the conflict of paradigms. Int Rev Neurobiol 1989; 31:297–353Crossref, Medline, Google Scholar
7. Rogers D: The motor disorders of severe psychiatric illness: a conflict of paradigms. Br J Psychiatry 1985; 147:221–223Crossref, Medline, Google Scholar
8. Kraepelin E: Manic-Depressive Insanity and Paranoia. Translated by Barclay RM, edited by Robertson GM. Edinburgh, E & S Livingstone, 1919Google Scholar
9. Fenton WS, Wyatt RJ, McGlashan TH: Risk factors for spontaneous dyskinesia. Arch Gen Psychiatry 1994; 51:643–650Crossref, Medline, Google Scholar
10. Turner T: Rich and mad in Victorian England. Psychol Med 1989; 19:29–44Crossref, Medline, Google Scholar
11. Kane JM, Smith JM: Tardive dyskinesia: rate and risk factors, 1959–1979. Arch Gen Psychiatry 1982; 39:473–481Crossref, Medline, Google Scholar
12. Kane JM, Woerner M, Lieberman J: Tardive dyskinesia: rate incidence and risk factors, in Dyskinesia Research and Treatment: Psychopharmacology Supplement 2. Edited by Casey DE, Chase T, Christensen AV, Gerlach J. Berlin, Springer, 1985, pp 72–78Google Scholar
13. Owens DGC, Johntone EC, Frith CD: Spontaneous disorders of involuntary movement. Arch Gen Psychiatry 1982; 39:643–650Crossref, Medline, Google Scholar
14. Chorfi M, Moussaoui D: Les schizophrenes jamais traités n’ont pas de movements anormaux type dyskinesie tardive. Encephale 1985; 11:263–265Medline, Google Scholar
15. McCreadie RG, Ohaeri JU: Movement disorder in never and minimally treated Nigerian schizophrenic patients. Br J Psychiatry 1994; 164:184–189Crossref, Medline, Google Scholar
16. McCreadie RG, Thara R, Kamath S, Padmavathy R, Latha S, Mathrubootham N, Menon MS: Abnormal movements in never- medicated Indian patients with schizophrenia. Br J Psychiatry 1996; 168:221–226Crossref, Medline, Google Scholar
17. Moussaoui D, Fenn D, Kadri N, Green C, Tilane A, Bentounsi B, Casey D, Hoffman D: Comparative studies of abnormal involuntary movements in never-treated vs treated populations with schizophrenia (abstract). Eur Psychiatry 1996; 11(suppl 4):170Google Scholar
18. Casey DE, Hansen TE: Spontaneous dyskinesias, in Neuropsychiatric Movement Disorders. Edited by Jeste DV, Wyatt RJ. Washington, DC, American Psychiatric Press, 1984, pp 67–95Google Scholar
19. Chatterjee A, Chakos M, Koreen A, Geisler S, Sheitman B, Woerner M, Kane JM, Alvir J, Lieberman JA: Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiatry 1995; 152:1724–1729Link, Google Scholar
20. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534–537Google Scholar
21. Schooler NR, Kane JM: Research diagnoses for tardive dyskinesia (letter). Arch Gen Psychiatry 1982; 39:486–487Medline, Google Scholar
22. Chakos MH, Alvir JMJ, Woerner MG, Koreen A, Geisler S, Mayerhoff D, Sobel S, Kane JM, Borenstein M, Lieberman JA: Incidence and correlates of tardive dyskinesia in first episode of schizophrenia. Arch Gen Psychiatry 1996; 53:313–319Crossref, Medline, Google Scholar
23. Woerner MG, Kane JM, Lieberman JA, Alvir JM, Bergmann KJ, Borenstein M, Schooler NR, Mukherjee S, Rotrosen J, Rubinstein M, Basavaraju N: The rate of tardive dyskinesia. J Clin Psychopharmacol 1991; 11:34–42Crossref, Medline, Google Scholar
24. Gardos G, Casey DE (eds): Tardive Dyskinesia and Affective Disorders. Washington, DC, American Psychiatric Press, 1984Google Scholar
25. Browne S, Roe M, Lane A, Gervin M, Morris M, Kinsella A, Larkin C, O’Callaghan E: Quality of life in schizophrenia: relationship to sociodemographic factors, symptomatology and tardive dyskinesia. Acta Psychiatr Scand 1996; 94:118–124Crossref, Medline, Google Scholar
26. O’Callaghan E, Larkin C, Kinsella A, Waddington JL: Obstetric complications, the putative familial-sporadic distinction, and tardive dyskinesia. Br J Psychiatry 1990; 157:578–584Crossref, Medline, Google Scholar
27. Waddington JL, Youssef HA, Dolphin C, Kinsella A: Cognitive dysfunction, negative symptoms, and tardive dyskinesia in schizophrenia. Arch Gen Psychiatry 1987; 44:907–912Crossref, Medline, Google Scholar
28. Waddington JL, O’Callaghan E, Buckley P, Madigan C, Redmond O, Stack PJ, Kinsella A, Larkin C, Ennis JT: Tardive dyskinesia in schizophrenia: relationship to minor physical anomalies, frontal lobe dysfunction and cerebral structure on magnetic resonance imaging. Br J Psychiatry 1995; 167:41–44Crossref, Medline, Google Scholar
29. Brown KW, White T: The influence of topography on the cognitive and psychopathological effects of tardive dyskinesia. Am J Psychiatry 1992; 149:1385–1389Link, Google Scholar
30. Waddington JL, O’Callaghan E, Larkin C, Kinsella A: Cognitive dysfunction in schizophrenia: organic vulnerability factor or state marker for tardive dyskinesia? Brain Cogn 1993; 23:56–70Google Scholar
31. Sandyk R, Kaye SR: The relationship of tardive dyskinesia to positive schizophrenia. Int J Neurosci 1991; 56:107–139Crossref, Medline, Google Scholar
32. Gureje O: Topographic subtypes of tardive dyskinesia in schizophrenic patients aged less than 60 years: relationship to demographic, clinical, treatment, and neuropsychological variables. J Neurol 1988; 51:1525–1530Google Scholar
33. Waddington JL, Youssef HA, Kinsella A: Cognitive dysfunction in schizophrenia followed up over 5 years, and its longitudinal relationship to the emergence of tardive dyskinesia. Psychol Med 1990; 20:835–842Crossref, Medline, Google Scholar
34. Wegner JT, Kane JM, Weinhold P, Woerner M, Kinon B, Lieberman J: Cognitive impairment in tardive dyskinesia. Psychiatry Res 1985; 16:331–337Crossref, Medline, Google Scholar
35. Rumberger RW: High school dropouts: a review of issues and evidence. Rev Educational Res 1987; 57:101–121Crossref, Google Scholar
36. Morgenstern H, Glazer WM: Identifying risk factors for tardive dyskinesia among long-term outpatients maintained with neuroleptic medications. Arch Gen Psychiatry 1993; 50:723–733Crossref, Medline, Google Scholar
37. Waddington JL, Youssef HA: Late onset involuntary movements in chronic schizophrenia: age related vulnerability to “tardive” dyskinesia independent of neuroleptic medication. Ir Med J 1985; 78:143–146Medline, Google Scholar
38. Smith JM, Kucharski LT, Oswald WT, Waterman LJ: Tardive dyskinesia: effect of age, sex, and criterion level of symptomatology on rate estimates. Psychopharmacol Bull 1979; 15:69–71Medline, Google Scholar
39. Smith JM, Kucharski LT, Oswald WT, Waterman LJ: A systematic investigation of tardive dyskinesia in inpatients. Am J Psychiatry 1979; 136:918–922Link, Google Scholar
40. Chouinard G, Annable L, Ross-Chouinard A, Nestoros JN: Factors related to tardive dyskinesia. Am J Psychiatry 1979; 136:79–83Link, Google Scholar
41. Smith JM, Dunn DD: Sex differences in the prevalence of severe tardive dyskinesia. Am J Psychiatry 1979; 136:1080–1082Link, Google Scholar



