Progressive Reduction of Temporal Lobe Structures in Childhood-Onset Schizophrenia
Abstract
OBJECTIVE: A previous cross-sectional study of brain morphology in childhood-onset schizophrenia indicated sparing of the temporal lobes from processes reducing total cerebral volume in this population. In the present study, subjects with childhood-onset schizophrenia and healthy subjects were rescanned at 2-year follow-up to determine whether this pattern of temporal lobe sparing persists with ongoing illness. METHOD: Anatomic brain magnetic resonance imaging scans were acquired for 10 adolescent patients with average onset of schizophrenia at 10.4 years (SD=1.7) and 17 healthy adolescents. Scans were obtained on initial admission and at 2-year follow-up by using identical equipment and measurement methodology. RESULTS: Schizophrenic subjects showed significantly greater decreases than healthy subjects in right temporal lobe, bilateral superior temporal gyrus and posterior superior temporal gyrus, right anterior superior temporal gyrus, and left hippocampal volumes during the follow-up interval. Decline in right posterior superior temporal gyrus was associated with high total scores on the Scale for the Assessment of Positive Symptoms at baseline and at follow-up. CONCLUSIONS: Progressive reduction of temporal lobe structures occurs with ongoing illness in childhood-onset schizophrenia.
Childhood-onset schizophrenia is a rare, severe form of schizophrenia that is associated with disruption of cognitive, linguistic, and social development well before the appearance of frank psychotic symptoms (1). An ongoing study of the neurobiology and clinical features of childhood-onset schizophrenia at the National Institute of Mental Health (NIMH) (2) has demonstrated abnormalities of eye tracking (3), autonomic function (4), and brain morphology (5) similar to those found for adult-onset schizophrenia. These observations are consistent with neurodevelopmental hypotheses, which propose that schizophrenia results from an early, static lesion that gives rise to typical symptoms as critical cerebral systems mature (6, 7). However, recent longitudinal magnetic resonance imaging (MRI) data from the NIMH childhood-onset schizophrenia cohort have indicated progressive increases in lateral ventricle volume and decreases in midsagittal thalamic area in childhood-onset schizophrenia at 2-year follow-up (8). These observations raise the possibility that brain abnormalities, at least in this rare, severely ill subgroup, may be progressive.
Previous longitudinal studies of brain morphology in schizophrenia have sampled only adult-onset cases and have focused primarily on ventricle size and ventricle-to-brain ratio (VBR). With some exceptions (9–13), most studies have not found evidence of greater pro~gressive increase in ventricle size or VBR in schizophrenia over intervals ranging from 2 to 9 years (14–20). However, in the majority of these studies, ventricle and brain size was estimated from single slices acquired with computed tomography (11, 12, 15–19), which can be inaccurate (21). The only previous longitudinal study of temporal lobe morphology in schizophrenia has now examined 50 patients and 20 comparison subjects with annual brain MRI over 4 to 5 years, finding no evidence of progressive change (13).
Cross-sectional postmortem and in vivo MRI studies of adult-onset schizophrenia have frequently reported reduced temporal lobe and medial temporal lobe volumes (22–26), although not without exceptions (27–31). Abnormal temporal lobe morphology in schizophrenia has been most frequently found on the left (23, 24, 32–34). Initial cross-sectional MRI examination of temporal lobe morphology in the NIMH childhood-onset schizophrenia sample (mean age at onset of psychosis=10.2 years) indicated relative sparing of temporal lobe structures (35). Specifically, after adjustment for the significantly smaller total cerebral volume of the schizophrenic subjects, superior temporal gyrus, posterior superior temporal gyrus, and temporal lobe tended to be relatively larger in the schizophrenic subjects, while medial temporal lobe structures showed no group differences.
In the present study, 10 adolescents with childhood-onset schizophrenia and 17 healthy adolescent comparison subjects were rescanned at 2-year intervals to determine whether sparing of temporal lobe structures persists in childhood-onset schizophrenia. Given previous observations of progressive increases in lateral ventricle volume and decreases in thalamic area in our childhood group, we hypothesized that temporal lobe structures of schizophrenic subjects would show relatively greater decreases in volume over time. Given previously observed relationships between reduced temporal lobe volumes and psychotic symptoms in cross-sectional studies of schizophrenic adults (24, 36–38), we further hypothesized that clinical state at 2-year follow-up, as measured by scores on the Scale for the Assessment of Positive Symptoms (SAPS) (39) and the Scale for the Assessment of Negative Symptoms (SANS) (40), would be correlated with reduction in the volume of temporal lobe and medial temporal lobe structures.
METHOD
Subjects
Schizophrenic children and adolescents were recruited nationally for an ongoing study of childhood-onset schizophrenia (41). Details of inclusion criteria and diagnostic assessment have been presented elsewhere (5, 35). All subjects had onset of schizophrenia by age 12 and were refractory to treatment with typical antipsychotics. For a parallel study of normal brain development, healthy pediatric subjects were recruited and screened (42). Individuals with physical, neurologic, or lifetime histories of psychiatric abnormalities, and those with first-degree relatives or greater than 20% of second-degree relatives with major psychiatric disorders, were excluded.
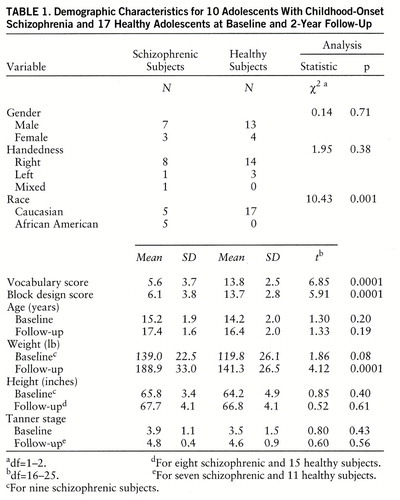
Both healthy and schizophrenic subjects agreed at the time of initial study to return for rescan every 2 years. Thus, 2-year rescan data were available from 10 patients and 17 healthy subjects. Mean age at onset of psychosis for these patients was 10.4 years (SD=1.7), and they had received an average of 23.3 months (SD=14.6) of neuroleptic treatment before study entry. At follow-up, all subjects were interviewed by using the same instruments used for the baseline assessment. Demographic data for both groups at baseline and 2-year follow-up are presented in table 1.
Parents of all subjects provided written informed consent, and subjects provided assent for participation in this study. This study was approved by the NIMH Institutional Review Board.
MRI Image Acquisition
All initial and follow-up scans were obtained on the same GE 1.5-T Signa magnetic resonance scanner, as described in detail elsewhere (42). Acquisitions of initial and 2-year rescan images for healthy and schizophrenic subjects were within 6 months of each other. Two-mm-thick contiguous slices were acquired in the coronal plane, and 1.5-mm-thick contiguous slices were acquired in the axial plane by using three-dimensional spoiled gradient recalled echo in the steady state (time to echo=5 msec, repetition time=24 msec, flip angle=45°, acquisition matrix=192×256, number of excitations=1, and field of view=24 cm2). To standardize head placement, vitamin E capsules, wrapped in gauze, were placed in the meatus of each ear, and a third capsule was taped to the lateral aspect of the left inferior orbital ridge. Subjects were aligned such that all three capsules appeared on the same axial slice of a multi-echo axial series. Comparison, with the Wilcoxon rank sums test, of the angle of a line drawn between the anterior and posterior commissure and the x-y plane indicated no differences in head position between groups or across time.
Image Analysis
To avoid bias related to rater “drift,” all scans were measured by a rater who was blind to subject diagnosis, age, and sex and to time of scan and were measured over the same 3-month period for total cerebral volume and volumes of temporal lobe and medial temporal lobe structures.
Quantification of total cerebral volume. Spatial orientation of the brain was standardized by using operator-selected midline anterior and posterior commissure points and the plane of the interhemispheric fissure. Brain matter was then separated from intracranial cavity by using software that employs an active surface template of a standard brain that is molded to fit the brain imaging data from specific subjects through successive iterations of an energy minimization function (43). Extracerebral tissue remaining in the resulting image was then removed by manual editing of each axial slice. This method provides a measure of right and left cerebral hemisphere volume excluding ventricles, brainstem, and cerebellum. Intra-rater and interrater reliabilities for 10 brains remeasured by the same and a second rater were (interclass correlation coefficient [ICC]) 0.94 and 0.89, respectively.
Temporal lobe and medial temporal lobe structures. Temporal lobe and medial temporal lobe structures were measured manually on sequential coronal slices by an experienced rater (A.C.V.), using NIH Image (44). Volumes were calculated by multiplying area measurements by slice thickness. The temporal stem was demarcated by a line connecting the most inferior point of the insular cisterns to the most lateral point of the basal cisterns above the hippocampus. The most posterior slice containing splenium of the corpus callosum demarcated the posterior extent of the temporal lobes (45). The superior temporal gyrus was identified by its gyral boundary and traced throughout its extent. The most posterior slice containing fibers of the fornix was designated as the posterior boundary of this structure (24). Anterior and posterior segments of the superior temporal gyrus were also identified, with the anterior segment ending at the most posterior slice before the appearance of the mamillary bodies. This slice was also designated as the posterior extent of the amygdala (24), while the first slice containing the mamillary bodies marked the anterior boundary of the hippocampus. The most posterior slice containing fibers of the fornix demarcated the posterior boundary of the hippocampus.
Ten brains remeasured by the rater for this study (A.C.V.) revealed intra-rater reliabilities (ICCs) of 0.99 for the temporal lobe, 0.97 for the superior temporal gyrus, 0.90 for the hippocampus, and 0.98 for the amygdala. Interrater reliabilities (ICCs) for 10 brains were 0.98 for the temporal lobe, 0.92 for the superior temporal gyrus, 0.87 for the hippocampus, and 0.86 for the amygdala.
Assessment of Symptoms
Clinical symptoms were rated with the SAPS and the SANS while patients were drug free during their baseline visit to NIH and at 2-year follow-up. Interrater reliabilities (ICCs), based on ratings for 10 patients by two sets of child psychiatrists at two points in the study (one rater consistent across both assessments), ranged between 0.87 and 0.91 for the SAPS and between 0.81 and 0.92 for the SANS.
Statistical Analyses
Group differences in demographic variables were assessed with chi-square analyses and t tests for independent samples. Total cerebral volume, temporal, and medial temporal lobe structures were analyzed by using repeated measures analysis of variance (ANOVA), with diagnosis as a between-subjects variable and side and time as within-subjects variables. To determine whether or not observed changes in temporal lobe volumes merely reflected changes in total cerebral volume during the follow-up interval, temporal and medial temporal lobe analyses were also performed through use of repeated measures analysis of covariance (ANCOVA), with total cerebral volume at baseline and 2-year follow-up as the covariate. Significant two- and three-way interactions (p<0.05) were plotted and further assessed by using Bonferroni post hoc t tests. Post hoc analyses of three-way interactions were examined by using Bonferroni post hoc t tests, with change scores in the numerators.
Within the schizophrenic group, exploratory correlation analyses that used Pearson's correlation coefficients were conducted to examine the relationship between change in temporal lobe morphology (baseline minus follow-up) and total SANS and total SAPS score during drug-free baseline and 2-year follow-up; change in total SANS and total SAPS score from drug-free baseline to 2-year follow-up (baseline minus follow-up); SAPS global rating of hallucinations, delusions, and thought disorder at follow-up (items 7, 20, and 34); WISC-R vocabulary subtest score at baseline; duration of illness; months of neuroleptic exposure before study entry; lifetime chlorpromazine equivalent exposure (calculated as previously described [5]); and change in lateral ventricle volume from baseline to 2-year follow-up (8).
All analyses were conducted through use of SAS (46) except for ANCOVA, which was conducted by using BMDP (47). All p values are two-tailed.
RESULTS
As can be seen in table 1, patients and healthy subjects did not significantly differ in gender, handedness, age, height, or Tanner stage at baseline or 2-year follow-up. However, the schizophrenic group included more African Americans, had significantly lower WISC-R vocabulary and block design subscale scores on study entry, and weighed more than healthy subjects at 2-year follow-up. Total cerebral volume was significantly smaller for the schizophrenic group at 2-year follow-up (schizophrenic subjects at baseline: mean=1111.5 cc, SD=123.1, at follow-up: mean=1060.3 cc, SD=140.8; healthy subjects at baseline: mean=1158.6 cc, SD=139.3, at follow-up: mean=1161.9 cc, SD=140.2). ANOVA indicated a significant diagnosis-by-time interaction (F=8.74, df=1,25, p=0.007), with schizophrenic subjects showing a greater bilateral decrease in total cerebral volume between baseline and 2-year follow-up. There were no other significant interactions; however, the right cerebrum was larger than the left across groups (F=5.21, df=1,25, p=0.03).
Status of Childhood-Onset Schizophrenic Subjects at 2-Year Follow-Up
At 2-year follow-up, eight of 10 subjects with schizophrenia were living at home, while one was maintained in a group home and one in a residential treatment center. Although most patients were more stable clinically at follow-up than at baseline, most continued to experience moderate psychotic symptoms. Mean total SAPS and total SANS scores at 2-year follow-up were 19.3 (SD=9.8) and 55.2 (SD=29.2), respectively. Seven of 10 patients met DSM-III-R criteria for schizophrenia at follow-up, while two met criteria for schizophrenia in partial remission, and one was in full remission. Two patients also met criteria for comorbid disorders at follow-up; one met criteria for autistic disorder and Tourette's disorder and another for Tourette's disorder and obsessive-compulsive disorder.
All patients received continuous antipsychotic pharmacotherapy during the interim period. Eight of 10 patients had been maintained on regimens of clozapine during the 2-year interim period, with an average daily dose of 301 mg (SD=104) at follow-up. The remaining two were taking 8 and 10 mg/day of risperidone, respectively. Monotherapy was used for five patients; adjunctive medications received by the remaining five patients included valproate, lithium, fluoxetine, and imipramine.
Temporal Lobe and Superior Temporal Gyrus
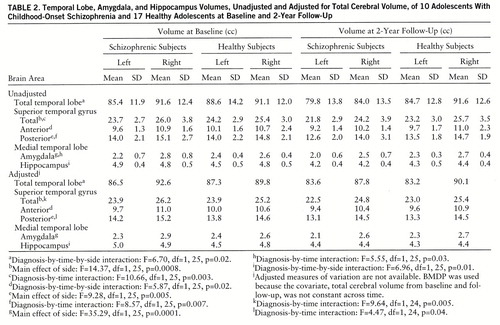
Mean volumes, unadjusted and adjusted for total cerebral volume, of temporal lobe structures for schizophrenic and healthy subjects at baseline and 2-year follow-up are shown in table 2.
A significant diagnosis-by-time-by-side interaction was observed for the total temporal lobe and is plotted in figure 1A. Post hoc testing indicated that on the right, volume decreases from baseline to follow-up were greater for schizophrenic subjects than for healthy subjects, while on the left, volume changes from baseline to follow-up did not differ between groups. On average, schizophrenic subjects experienced an 8.3% decrease in right temporal lobe volume over the follow-up interval. Right temporal lobe volume loss of at least this magnitude was experienced by five of 10 schizophrenic and no healthy subjects.
Significant diagnosis-by-time interactions were observed for the total superior temporal gyrus and its posterior segment, with post hoc tests indicating a significant decrease in volume from baseline to follow-up for schizophrenic subjects but not for healthy subjects for both structures. On average, schizophrenic subjects experienced a 7.4% decrease in superior temporal gyrus volume and an 8.6% decrease in posterior superior temporal gyrus volume over the follow-up interval. At least this magnitude of volume loss for the superior temporal gyrus was experienced by eight schizophrenic subjects and four healthy subjects and for the posterior superior temporal gyrus was experienced by six schizophrenic subjects and three healthy subjects.
A significant diagnosis-by-time-by-side interaction was observed for the anterior superior temporal gyrus (figure 1B), with post hoc tests indicating that on the right, volume decreases from baseline to follow-up were greater for schizophrenic subjects than for healthy subjects, while on the left, volume changes from baseline to follow-up did not differ between groups. On average, schizophrenic subjects experienced a 6.4% decrease in right anterior superior temporal gyrus volume over the follow-up interval. Right anterior superior temporal gyrus volume loss of at least this magnitude was observed in eight schizophrenic subjects and eight healthy subjects.
Medial Temporal Lobe
Amygdala volumes were significantly larger on the right for both groups. Although ANOVA indicated a significant diagnosis-by-time interaction, reflecting greater decreases in amygdala volume from baseline to follow-up for schizophrenic subjects, this interaction did not remain significant following adjustment for total cerebral volume.
For the hippocampus, a significant diagnosis-by-time-by-side interaction was observed and is plotted in figure 1C. Post hoc testing indicated that on the right, volumes decreased from baseline to follow-up by an equal amount in schizophrenic and healthy subjects, while on the left, volumes decreased significantly more in the schizophrenic subjects. On average, schizophrenic subjects experienced a 14.3% decrease in left hippocampal volume over the follow-up interval. Left hippocampal volume loss of at least this magnitude was found in four schizophrenic subjects and one healthy subject.
The five schizophrenic subjects receiving adjunctive medications during the follow-up period exhibited a pattern of reduction in temporal lobe volumes over the follow-up interval that was similar to that observed in the schizophrenic group as a whole and in the schizophrenic subjects receiving monotherapy. In addition, the two schizophrenic subjects with comorbid diagnoses at follow-up were similar to the schizophrenic group as a whole in pattern of reduction of temporal lobe volumes.
Clinical Status and Changes in Temporal Lobe Morphology Within the Schizophrenic Group
Total SAPS score at baseline was significantly positively correlated with change in superior temporal gyrus and posterior superior temporal gyrus volumes (r=0.75, N=10, p=0.01 and r=0.70, N=10, p=0.02, respectively), indicating that greater positive symptoms at baseline predicted greater reduction in the volumes of these structures. Similarly, total SAPS score at 2-year follow-up was significantly correlated with change in posterior superior temporal gyrus volume (r=0.62, N=10, p=0.05). Greater decreases in the volume of this structure during the follow-up interval were associated with greater positive symptoms at follow-up. Finally, SANS total score at baseline and SAPS global rating of delusions at follow-up were both significantly correlated with change in hippocampal volume (r=0.65, N=10, p=0.04 and r=0.67, N=10, p=0.03), indicating that greater negative symptoms at baseline and greater delusions at follow-up were associated with greater decreases in hippocampal volume over the follow-up interval. SAPS global ratings of hallucinations and thought disorder at follow-up, SANS total score at follow-up, WISC-R vocabulary subtest score, months of neuroleptic exposure, lifetime chlorpromazine equivalent exposure, and change in lateral ventricle volume over the follow-up interval were not significantly correlated with change in temporal lobe morphology within the schizophrenic group.
Significant correlations were further evaluated by repeating these specific analyses with the left and right volumes of the hippocampus, superior temporal gyrus, and posterior superior temporal gyrus separately. All significant correlations involved the right side of structures only. Specifically, change in right hippocampal volume was significantly positively correlated with SAPS global rating of delusions at follow-up (r=0.70, N=10, p=0.02), while change in right posterior superior temporal gyrus volume was significantly positively correlated with total SAPS score at baseline (r=0.69, N=10, p=0.03) and total SAPS score at 2-year follow-up (r=0.78, N=10, p=0.008). The relationship between change in posterior superior temporal gyrus volume and SAPS score at 2-year follow-up is plotted in figure 1D. Total SANS score at baseline was positively correlated with change in right hippocampal volume (r=0.62, N=10, p=0.06), and total SAPS score at baseline was positively correlated with change in right superior temporal gyrus volume (r=0.62, N=10, p=0.06).
DISCUSSION
In the present study, progressive reductions in the volume of temporal lobe, superior temporal gyrus, and hippocampus were observed that were more pronounced in adolescents with childhood-onset schizophrenia than in healthy subjects, even after control for the 4.6% decrease in total cerebral volume sustained by childhood-onset schizophrenic subjects over the same time interval. Comparable decline in total cerebral volume over time has been observed in adult-onset schizophrenia (13) and, in the present study, was not correlated with age at onset, duration of illness, neuroleptic exposure, or total SANS or SAPS scores at baseline or 2-year follow-up.
Previously reported relationships between positive symptoms of psychosis and smaller superior temporal gyrus volumes in adult schizophrenia have been specific to the left side (24, 25). In childhood-onset schizophrenic subjects, decreases in right posterior superior temporal gyrus volume were associated with higher SAPS scores at baseline and were strongly associated with higher SAPS scores at 2-year follow-up. Although volume decreases of the hippocampus were greater for schizophrenic subjects than for healthy subjects on the left, volume reduction of the right hippocampus was associated with high SAPS global ratings of delusions at 2-year follow-up. Positive psychotic symptoms have been found to be related to reduction of both right and left amygdala-hippocampus volumes in adult schizophrenia (36).
These findings suggest that as hypothesized, the previously observed sparing of temporal lobe structures from the process or processes that had already reduced the volume of other cortical regions in this group by the time of study entry does not persist. As observed for decreases in midsagittal thalamic area in this group during the same follow-up interval (8), decline in volumes of temporal lobe structures exceeded the decline in total cerebral volume during this interval, particularly for the hippocampus. Previous exposure to typical antipsychotics was not associated with temporal lobe volumes at baseline (43) or with reduction in temporal lobe structures during follow-up, suggesting that neither the initial sparing nor the subsequent decrease in temporal lobe volumes was related to exposure to these medications. However, all of these schizophrenic subjects were switched from typical to atypical antipsychotic therapy shortly after study entry, a time point coincident with the shift from a sparing to a nonsparing pattern of temporal lobe morphologic abnormalities. While this potentially confounds interpretation of the observed brain morphologic changes, it should be noted that volume reductions associated with atypical antipsychotic therapy have only been observed for basal ganglia structures in patients previously receiving typical antipsychotics, which appear to induce increases in basal ganglia volumes (48, 49). Furthermore, many of the previous cross-sectional reports of reduced temporal lobe structures in adult schizophrenia have involved patients treated with typical antipsychotics (23, 24, 33).
While a medication effect cannot be ruled out, these findings, together with the greater progressive increase in ventricle volume and decrease in midsagittal thalamic area for the same group over the same time period, suggest a nonstatic, continuous process leading to and/or exacerbating prior brain abnormalities in childhood-onset schizophrenia (13, 50). As others have proposed, this process may occur only during mid to late adolescence and thus may not be detectable in most longitudinal studies of adults with schizophrenia (50–52). Alternatively, such progressive changes may be specific to childhood-onset schizophrenia and other subgroups of very ill patients. While most of the patients in this study were living with their families in the community at follow-up, all continued to experience psychotic symptoms despite ongoing pharmacotherapy. Timing of the appearance of brain morphologic abnormalities in patients with more typical early adult age at onset and episodic course may be quite different (50).
The potential neurobiological underpinnings of the progressive changes observed here are unknown but may include loss of neuropil, as might result from excessive synaptic pruning (53), premature apoptosis (programmed cell death) of neurons or glia, and/or abnormal loss of neurons (degeneration). Planned regional gray/white segmentation of this longitudinal MRI data set may clarify whether progressive volume loss in temporal lobe structures is restricted to gray matter, as appears to be the case for volume decreases of the total cerebrum (54). The strong relationships observed between greater psychotic symptoms at baseline and at follow-up and greater decrements in the volumes of temporal lobe structures are consistent with the process leading to reduction in temporal lobe volumes being disease related.
The present study involved a relatively small group size, and multiple statistical tests were performed, increasing the risk of type I error. Firm conclusions must await replication of these findings with a larger group. While the significant group differences in weight at 2-year follow-up potentially complicate interpretation of these findings, this difference most likely reflects the appetite-stimulating properties of the atypical antipsychotic medications that the schizophrenic subjects received during the follow-up interval. Consistent with this, the groups did not differ in height. The group differences in race also potentially confound these findings; however, there were no significant differences between African American and Caucasian schizophrenic patients in changes in total cerebral, temporal lobe, amygdala, or hippocampal volumes from baseline to 2-year follow-up. This imbalance in the healthy subject group is being corrected. While schizophrenic and healthy subjects differed significantly in intellectual functioning, previous observations from adult schizophrenia samples suggest that these differences are probably disease related (55).
In contrast to the slight decreases in some temporal lobe structures observed for healthy subjects in the present study, our previous cross-sectional examination of temporal lobe development in 99 healthy children ages 4 to 18 indicated increases in left amygdala for males and right hippocampus for females (56). However, these cross-sectional data are characterized by a high degree of variability that diminishes statistical power to detect nonlinear developmental curves. Thus, it is possible that hippocampal and amygdala volumes show a net increase in healthy children between ages 4 and 18, while across smaller age ranges the volumes may actually be decreasing.
Finally, the present study does not address the question of continued progression of volume loss for temporal lobe structures. Ongoing every-2-year clinical follow-up with MRI brain rescan is being conducted to determine, in particular, whether volume decrements continue to the point where temporal lobe structures are relatively smaller in childhood-onset schizophrenic subjects, as has been observed in adult patients.
 |
 |
Received July 8, 1997; revision received Nov. 18, 1997; accepted Nov. 25, 1997. From the Child Psychiatry Branch, NIMH. Address reprint requests to Dr. Jacobsen, Yale University School of Medicine, Department of Psychiatry, VA Connecticut Health Care System/116A, 950 Campbell Ave., West Haven, CT 06516. The authors thank Drs. Jean A. Frazier, Kathleen McKenna, Javad Alaghband-Rad, and Charles T. Gordon for their assistance with this study.
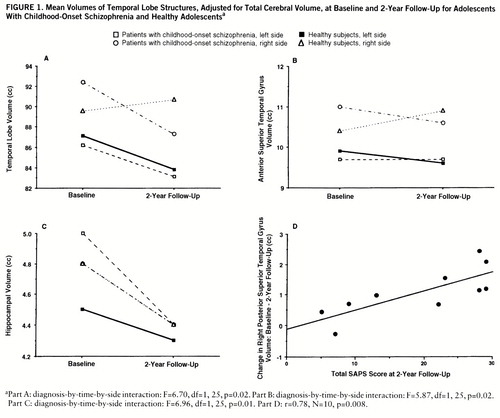
FIGURE 1. Mean Volumes of Temporal Lobe Structures, Adjusted for Total Cerebral Volume, at Baseline and 2-Year Follow-Up for Adolescents With Childhood-Onset Schizophrenia and Healthy Adolescentsa
aPart A: diagnosis-by-time-by-side interaction: F=6.70, df=1,25, p=0.02. Part B: diagnosis-by-time-by-side interaction: F=5.87, df=1,25, p=0.02. Part C: diagnosis-by-time-by-side interaction: F=6.96, df=1,25, p=0.01. Part D: r=0.78, N=10, p=0.008.
1 Alaghband-Rad J, McKenna K, Gordon CT, Albus KE, Hamburger SD, Rumsey JM, Frazier JA, Lenane MC, Rapoport JL: Childhood-onset schizophrenia: the severity of premorbid course. J Am Acad Child Adolesc Psychiatry 1995; 34:1273–1283Crossref, Medline, Google Scholar
2 Jacobsen LK, Rapoport JL: Childhood onset schizophrenia: implications of clinical and neurobiological research. J Child Psychol Psychiatry (in press)Google Scholar
3 Jacobsen LK, Hong WL, Hommer DW, Castellanos FX, Frazier JA, Giedd JN, Gordon CT, Karp BI, McKenna K, Rapoport JL: Smooth pursuit eye movements in childhood-onset schizophrenia: comparison with ADHD and normal controls. Biol Psychiatry 1996; 40:1144–1154Crossref, Medline, Google Scholar
4 Zahn TP, Jacobsen LK, Gordon CT, McKenna K, Frazier JA, Rapoport JL: Autonomic nervous system markers of psychopathology in childhood onset schizophrenia. Arch Gen Psychiatry 1997; 54:904–912Crossref, Medline, Google Scholar
5 Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kaysen D, King AC, Rajapakse JC, Lenane MC, McKenna K, Jacobsen LK, Gordon CT, Breier A, Rapoport JL: Brain anatomic magnetic resonance imaging in childhood onset schizophrenia. Arch Gen Psychiatry 1996; 53:617–624Crossref, Medline, Google Scholar
6 Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660–669Crossref, Medline, Google Scholar
7 Murray RM, Lewis SW: Is schizophrenia a neurodevelopmental disorder? Br Med J 1987; 295:681–682Google Scholar
8 Rapoport JL, Giedd J, Kumra S, Jacobsen LK, Smith A, Lee P, Nelson J, Hamburger S: Childhood onset schizophrenia: pro~gressive ventricular change during adolescence. Arch Gen Psychiatry 1997; 54:897–903Crossref, Medline, Google Scholar
9 DeLisi LE, Tew W, Xie S, Hoff AL, Sakuma M, Kushner M, Lee G, Shedlack K, Smith AM, Grimson R: A prospective follow-up study of brain morphology and cognition in 1st episode schizophrenic patients: preliminary findings. Biol Psychiatry 1995; 38:349–360Crossref, Medline, Google Scholar
10 Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A: Longitudinal analysis of MRI brain volumes in schizophrenia (abstract). Schizophr Res 1997; 24:152Crossref, Google Scholar
11 Kemali D, Maj M, Galderisi S, Milici N, Salvati A: Ventricle-to-brain ratio in schizophrenia: a controlled follow-up study. Biol Psychiatry 1989; 26:753–756Crossref, Medline, Google Scholar
12 Woods BT, Yurgelun-Todd D, Benes FM, Frankenburg FR, Pope HG, McSparren J: Progressive ventricular enlargement in schizophrenia: comparison to bipolar affective disorder and correlation with clinical course. Biol Psychiatry 1990; 27:341–352Crossref, Medline, Google Scholar
13 DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R: Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res Neuroimaging 1997; 74:129–140Crossref, Medline, Google Scholar
14 Degreef G, Ashtari M, Wu H, Borenstein M, Geisler S, Lieberman J: Follow up MRI study in first episode schizophrenia. Schizophr Res 1991; 5:183–210Crossref, Medline, Google Scholar
15 Jaskiw GE, Juliano DM, Goldberg TE, Hertzman M, Urow-Hamell E, Weinberger DR: Cerebral ventricular enlargement in schizophreniform disorder does not progress: a seven year follow-up study. Schizophr Res 1994; 14:23–28Crossref, Medline, Google Scholar
16 Illowsky BP, Juliano DM, Bigelow LB, Weinberger DR: Stability of CT scan findings in schizophrenia: results of an 8 year follow-up study. J Neurol Neurosurg Psychiatry 1988; 51:209–213Crossref, Medline, Google Scholar
17 Nasrallah HA, Olson SC, McCalley-Whitters M, Chapman S, Jacoby CG: Cerebral ventricular enlargement in schizophrenia: a preliminary follow-up study. Arch Gen Psychiatry 1986; 43:157–159Crossref, Medline, Google Scholar
18 Vita A, Sacchetti E, Valvassori G, Cazzullo CL: Brain morphology in schizophrenia: a 2- to 5-year CT scan follow-up study. Acta Psychiatr Scand 1988; 78:618–621Crossref, Medline, Google Scholar
19 Hoffman WF, Ballard L, Turner EH, Casey DE: Three-year follow-up of older schizophrenics: extrapyramidal syndromes, psychiatric symptoms, and ventricular brain ratio. Biol Psychiatry 1991; 30:913–926Crossref, Medline, Google Scholar
20 Sponheim SR, Iacono WG, Beiser M: Stability of ventricular size after the onset of psychosis in schizophrenia. Psychiatry Res Neuroimaging 1991; 40:21–29Crossref, Medline, Google Scholar
21 Mayhew TM, Gundersen HJG: “If you assume, you can make an ass out of u and me”: a decade of the disector for stereological counting of particles in 3D space. J Anat 1996; 188:1–15Medline, Google Scholar
22 Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE: The hippocampus and parahippocampus in schizophrenic, suicide, and control brains. Arch Gen Psychiatry 1990; 47:1029–1034Crossref, Medline, Google Scholar
23 Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR: Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med 1990; 322:789–794Crossref, Medline, Google Scholar
24 Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW: Abnormalities of the left temporal lobe and thought disorder in schizophrenia. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
25 Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyrus volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Link, Google Scholar
26 Stefanis N, Yakeley J, Frangou S, Sharma T, O'Connell P, Morgan K, Murray R: Pregnancy and birth complications (PBC)-associated hippocampal volume reduction in sporadic schizophrenia (abstract). Schizophr Res 1997; 24:157Crossref, Google Scholar
27 Flaum M, Swayze VW II, O'Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC: Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry 1995; 152:704–714Link, Google Scholar
28 Heckers S, Heinsen H, Heinsen YC, Beckmann H: Limbic structures and lateral ventricle in schizophrenia: a quantitative postmortem study. Arch Gen Psychiatry 1990; 47:1016–1022Crossref, Medline, Google Scholar
29 Zipursky RB, Marsh L, Lim KO, DeMent S, Shear PK, Sullivan EV, Murphy GM, Csernansky JG, Pfefferbaum A: Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol Psychiatry 1994; 35:501–516Crossref, Medline, Google Scholar
30 DeLisi LE, Hoff AL, Schwartz JE, Shields GW, Halthore SN, Gupta SM, Henn FA, Anand AK: Brain morphology in first-episode schizophrenic-like psychotic patients: a quantitative magnetic resonance imaging study. Biol Psychiatry 1991; 29:159–175Crossref, Medline, Google Scholar
31 DeLisi LE, Stritzke P, Riordan H, Holan V, Boccio A, Kushner M, McClelland J, Van Eyl O, Anand A: The timing of brain morphological changes in schizophrenia and their relationship to clinical outcome. Biol Psychiatry 1992; 31:241–254Crossref, Medline, Google Scholar
32 Johnstone EC, Owens DGC, Crow TJ, Frith CD, Alexandropolis K, Bydder G, Colter N: Temporal lobe structure as determined by nuclear magnetic resonance in schizophrenia and bipolar affective disorder. J Neurol Neurosurg Psychiatry 1989; 52:736–741Crossref, Medline, Google Scholar
33 Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F: Brain morphology and schizophrenia: a magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry 1992; 49:921–926Crossref, Medline, Google Scholar
34 Bogerts B, Ashtari M, Degreef G, Alvir JMJ, Bilder RM, Lieberman JA: Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res 1990; 35:1–13Crossref, Medline, Google Scholar
35 Jacobsen LK, Giedd JN, Vaituzis AC, Hamburger SD, Rajapakse JC, Frazier JA, Kaysen D, Lenane MC, McKenna K, Gordon CT, Rapoport JL: Temporal lobe morphology in childhood-onset schizophrenia. Am J Psychiatry 1996; 153:355–361; correction, 1996; 153:851Google Scholar
36 Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Ler~ner G, Johns C, Masiar S: Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol Psychiatry 1993; 33:236–246Crossref, Medline, Google Scholar
37 Flaum M, O'Leary DS, Swayze VW II, Miller DD, Arndt S, Andreasen NC: Symptom dimensions and brain morphology in schizophrenia and related psychotic disorders. J Psychiatr Res 1995; 29:261–276Crossref, Medline, Google Scholar
38 Menon RR, Barta PE, Aylward EH, Richards SS, Vaughn DD, Tien AY, Harris GJ, Pearlson GD: Posterior superior temporal gyrus in schizophrenia: grey matter changes and clinical correlates. Schizophr Res 1995; 16:127–135Crossref, Medline, Google Scholar
39 Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
40 Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
41 Kumra S, Frazier JA, Jacobsen LK, McKenna K, Gordon CT, Hamburger SD, Smith AK, Albus KE, Alaghband-Rad J, Lenane MC, Rapoport JL: Childhood-onset schizophrenia: a double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry 1996; 53:1090–1097Crossref, Medline, Google Scholar
42 Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kor~uch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapo~port JL: Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex 1996; 6:551–560Crossref, Medline, Google Scholar
43 Snell JW, Merickel MB, Ortega JM, Goble JC, Brookeman JR, Kassell NF: Boundary estimation of complex objects using hierarchical active surface templates. Pattern Recognition 1995; 28:1599–1609Crossref, Google Scholar
44 Rasband W: Image 1.6. Bethesda, Md, National Institutes of Health, 1993 (public domain)Google Scholar
45 Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JMJ, Snyder PJ, Lieberman JA: Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry 1994; 151:1437–1447Link, Google Scholar
46 SAS Language and Procedures: Usage, version 6.07. Cary, NC, SAS Institute, 1989Google Scholar
47 BMDP Statistical Software I: BMDP. Berkeley, University of California Press, 1990Google Scholar
48 Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M: Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet 1995; 345:456–457Crossref, Medline, Google Scholar
49 Frazier JA, Giedd JN, Kaysen D, Albus K, Hamburger S, Alaghband-Rad J, Lenane MC, McKenna K, Breier A, Rapoport JL: Childhood-onset schizophrenia: brain MRI rescan after 2 years of clozapine maintenance treatment. Am J Psychiatry 1996; 153:564–566Link, Google Scholar
50 DeLisi LE: Is schizophrenia a lifetime disorder of brain plasticity, growth and aging? Schizophr Res 1997; 23:119–129Google Scholar
51 Keshavan MS: Neurodevelopment and schizophrenia: quo vadis? in Neurodevelopment and Adult Psychopathology. Edited by Keshavan MS, Murray RM. Cambridge, England, Cambridge University Press (in press)Google Scholar
52 Woods BT, Yurgelun-Todd D, Goldstein JM, Seidman LJ, Tsuang MT: MRI brain abnormalities in chronic schizophrenia: one process or more? Biol Psychiatry 1996; 40:585–596Google Scholar
53 Feinberg I: Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res 1982; 17:319–334Google Scholar
54 Giedd JN, Castellanos FX, Rajapakse JC, Jacobsen LK, Frazier JA, Hamburger SD, Rapoport JL: Quantitative analysis of gray matter volumes in childhood-onset schizophrenia and attention deficit/hyperactivity disorder. Abstracts of the Society for Neuroscience 1996; 22:1166Google Scholar
55 Aylward E, Walker E, Bettes B: Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull 1984; 10:430–459Crossref, Medline, Google Scholar
56 Giedd JN, Vaituzis AC, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Hamburger SD, Rapoport JL: Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18. J Comp Neurol 1996; 366:223–230Crossref, Medline, Google Scholar



